We are proud to announce the relaunch of the SafeTPen™ Class IIa Medical Device demonstrating our continued commitment to regulatory compliance and patient safety
While the SafeTPen™ itself remains unchanged in its design, it has undergone a more rigorous Conformity Assessment required to meet the enhanced requirements of the Medical Devices Regulation (MDR) (EU) 2017/745.







SafeTPen™ FAQs
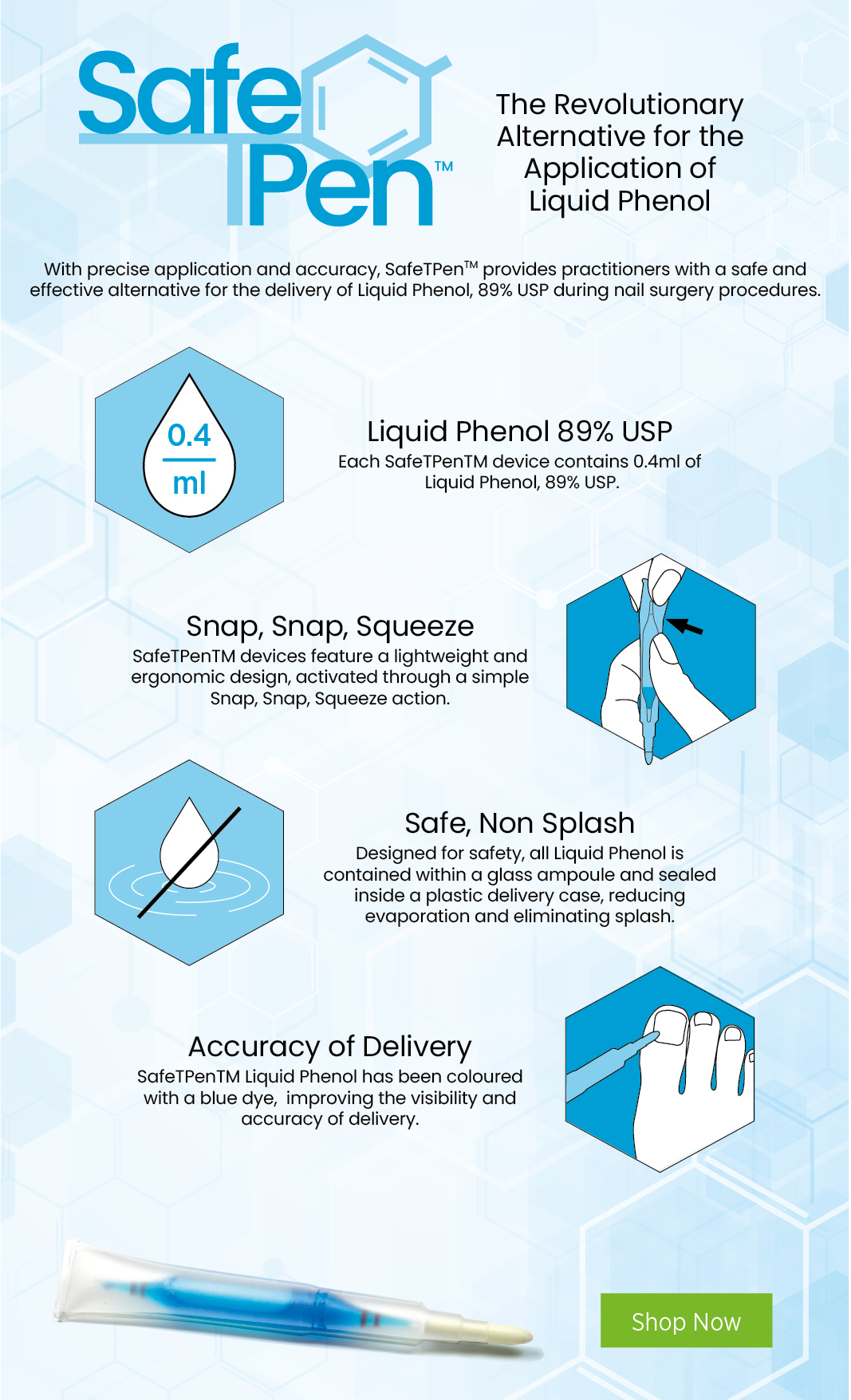
- Easy to use
- Reduces possibility of a phenol spill
- Controlled amount delivered to each patient
- A tapered tip to assist with more accurate delivery of phenol to the nail matrix
- Readily visible phenol through incorporation of medical dye
A blue dye has been blended to the phenol, so that it:
- Allows the practitioner to ensure the application of phenol is to the matrix
- Helps a practitioner see more clearly where the phenol is being applied during surgery, reducing the potential of phenol to be administered to surrounding tissue by mistake and causing the potential of burning intact skin
- You can see if the ampoule containing the phenol has been damaged prior to use
- Contains 100% more phenol than current market brands
- To prevent spillage and excessive use of Phenol during the nail ablation
- To reduce the risk of cross infection when undertaking the matrix ablation on a Bi-lateral partial nail avulsion
- Phenol within a glass bottle is a more stable product, reducing evaporation and absorption of moisture from the atmosphere which is a common occurrence when encased in a plastic tube/bottle. Because the liquid phenol is stored within a glass ampoule in SafeTPen™, we have been able to give the practitioner a longer shelf life on the product.
- So you can identify before opening the packaging if there has been leakage of phenol during transportation
- The manufacturing costs have increased, however what we are providing is a safer delivery system to you and your patient when undertaking nail surgery procedures.
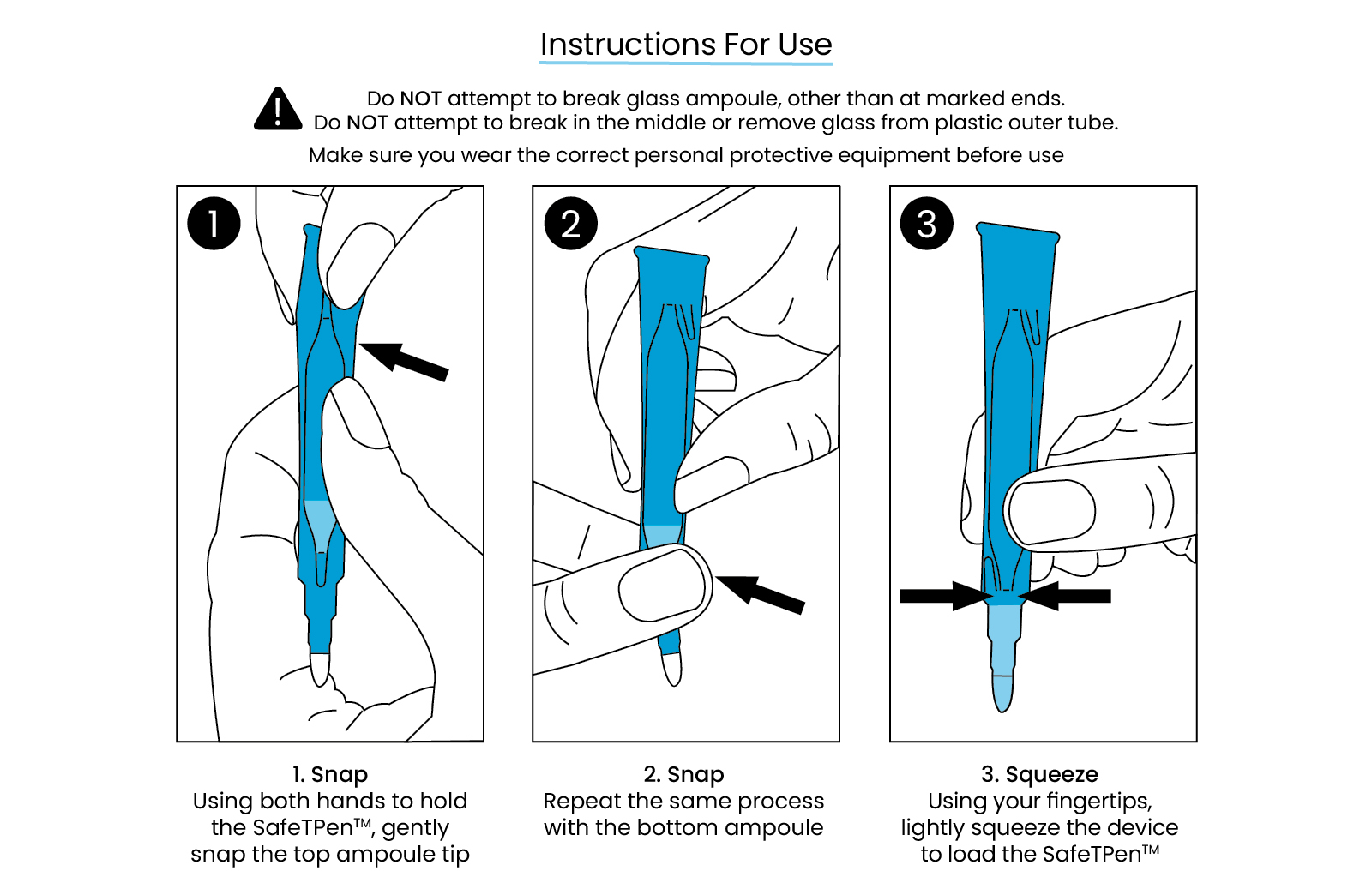
- The risk of a cut is minimal due to the glass ampoule remaining inside of the strong plastic delivery casing when breaking the ampoule
- Ensuring that the polyester tip remains in situ will prevent the glass tip from exuding from the tube and also the potential for increased phenol flow rate
- There is minimal risk of phenol leakage during transport due to the inner glass ampoule, plastic delivery device and the packaging, making it arguably the safest product on the market
- SafeTPen™ has been tried and tested for breakages and has withstood being dropped from a height of 4m and thrown a distance of 10m
- If for any reason there has been an issue with the ampoule breaking you will be able to quickly identify this through the blue dye being within the plastic delivery case, therefore we recommend you dispose of it in the medical waste. Don’t open the outer packet
- Dispose of it straight away in the medical waste – Don’t open the paper packet it is contained in
- Wear PPE to protect yourself
- Put absorbent swabs over the spillage, so that the phenol is absorbed / collected off the surface and dispose of these swabs in the medical waste bin
- Wash the surface with water, don’t use water spray as this could make the phenol an aerosol, disposing of any towel or swabs to clean the water up in the medical waste
- Smell associated with phenol will always be present, however with SafeTPen™ you aren’t decanting the phenol from a multi-use bottle to a galli-pot therefore there will be minimal fumes released
- Place the SafeTPen™ at the end of the procedure back into the clear plastic packaging and seal across the top
- SafeTPen™ is disposed of in the medical waste as per local guidelines and protocols.